BGU et Technion (Israël) : l'énergie solaire pour produire du carburant hydrogène commercial

[:fr]
Les constructeurs automobiles cherchent activement à développer des véhicules fonctionnant à l’hydrogène, considéré comme efficace et respectueux de l’environnement et qui, contrairement aux véhicules électriques, permet un ravitaillement rapide en carburant et une autonomie accrue.
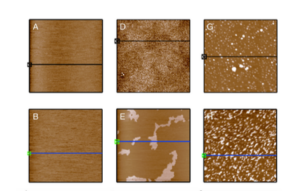
Cette nouvelle étude constitue donc une avancée décisive dans la compréhension du mécanisme de la division de l’eau. Les chercheurs dirigés par les Drs Arik Yochelis et Iris Visoly-Fisher de l’Université Ben Gourion du Néguev (BGU) et les chercheurs du Technion ont découvert le mécanisme chimique qui se produit lors de la scission photochimique du peroxyde d’hydrogène (H2O2) par photo-électrolyse de l’oxyde de fer.
Selon Ezra Banoun*, expert en énergies renouvelables et membre du comité scientifique d’Israël Science Info : « Des chercheurs ont craqué le mécanisme chimique qui se produit lors du fractionnement photochimique du peroxyde d’hydrogène (H2O2) sur les électrodes de la photo-oxyde de fer. Le peroxyde d’oxygène (eau oxygène) peut-être électrolysé par des électrodes de »photo-oxyde de fer ». En fait, la combinaison du module photovoltaïque pour capter l’énergie solaire et les cellules électrolytiques pour décomposer l’eau est de loin le système le plus attrayant et le plus simple. L’utilisation de l’énergie solaire dans les procédés d’électrolyse s’avère être la plus viable et la plus protectrice de l’environnement. Comme l’électricité à courant continu générée par le panneau photovoltaïque convient parfaitement aux systèmes d’électrolyse, la majorité des systèmes produisant de l’énergie à partir du soleil utilise des systèmes photovoltaïques pour la génération d’électricité pour l’électrolyse de l’eau. Il semble que l’innovation est la photo-électrolyse du peroxyde d’oxygène et non de l’eau suivant un procédé innovateur ».

Selon les chercheurs, cette découverte pourrait avoir un impact significatif sur les efforts visant à remplacer les carburants à base de carbone par des carburants à base d’hydrogène, plus écologiques. « Bien que l’on sache depuis des décennies que la production d’hydrogène ne dégageant pas de gaz à effet de serre nécessite la décomposition de molécules d’eau (H2O) en éléments constitutifs (deux atomes d’hydrogène et un atome d’oxygène), ce processus a toujours demandé plus d’énergie que ce qui avait été récupéré à la fin du processus. En conséquence, cela n’a jamais été commercialement viable », expliquent les chercheurs.
Le processus est commercialement viable

Pour relever ce défi, les chercheurs ont pour la première fois révélé avec succès la réaction chimique fondamentale présente dans l’énergie solaire qui pourrait constituer le chaînon manquant pour générer l’électricité nécessaire à la réalisation de ce processus. Cela permettrait au processus de se dérouler naturellement au lieu de s’appuyer sur de grandes quantités de sources d’énergie artificielles ou de métaux précieux pour catalyser la réaction. Après des années d’expériences difficiles au cours desquelles le laboratoire du Pr Avner Rothschild du Technion n’a pu surmonter l’obstacle, il a approché les Drs. Yochelis et Visoly-Fisher pour collaborer et tenter de trouver la pièce manquante du puzzle.
Une percée scientifique

«Au-delà de la percée scientifique, nous avons montré que le mécanisme de réaction photo-électrochimique appartient à une famille de réactions chimiques pour laquelle le Pr Gerhard Ertl a reçu le prix Nobel de chimie il y a une décennie. Notre découverte ouvre de nouvelles stratégies pour les processus photochimiques », déclare le Dr Yochelis de l’Institut de recherche sur le désert Jacob Blaustein de la BGU et du Département de l’énergie solaire et de physique environnementale (YDSEEP) Alexandre Yersin.
L’étude est financée par des subventions de recherche du ministère israélien des Infrastructures nationales, de l’Énergie et de l’Eau et du ministère israélien de la Science et de la Technologie.
Publication dans Nature Communications
Ezra Charles BANOUN, israélien, est manager, ingénieur, entrepreneur, développeur en technologie et formateur. Ezra a cumulé 34 années d’expérience de Direction Générale d’entreprises de traitement des eaux à partir de technologies avancées et 22 années d’expérience de développement de technologies originales pour permettre la production contrôlée d’eaux traitées à un niveau quasi-potable.
Ezra est aujourd’hui un expert reconnu en Israël dans le domaine de la gestion des ressources et du traitement des eaux. Il est aussi maitre de conférences à l’Institut International Universitaire de Management de Galilée dans les domaines de la gouvernance des ressources en eaux, de la lutte contre la désertification et de la reconquête du désert et du développement durable. Ezra est aussi auteur de nombreux articles pour des sites d’information, à la radio ou dans les journaux.
Contact : ezrabanoun@gmail.com
[:en]A new study presents a breakthrough toward understanding the mechanism of splitting water. Researchers led by Dr. Arik Yochelis and Dr. Iris Visoly-Fisher of Ben-Gurion University of the Negev and the Technion, have cracked the chemical mechanism that occurs during the photochemical splitting of hydrogen peroxide (H2O2) over iron-oxide photo-electrodes.
The discovery, they say, could have a significant impact on efforts to replace carbon-based fuels with more environmentally friendly hydrogen fuels. Car manufacturers seek to develop hydrogen-powered vehicles that are considered efficient and environmentally friendly and that, unlike electric vehicles, allow for fast refueling and extended mileage.
The researchers say that although it has been well-known for decades that production of hydrogen that does not emit greenhouse gases requires the splitting of water molecules (H2O) into the elements from which they are composed (two hydrogen atoms and one oxygen atom), that process has always demanded more energy than was gained back at the end of the process. As a result, it has never been commercially viable.
To conquer this challenge, the researchers were the first team to successfully reveal the fundamental chemical reaction present in solar power that could form the missing link to generate the electricity necessary to accomplish this process. That would allow the process to unfold naturally instead of relying on large amounts of man-made energy sources or precious metals to catalyze the reaction.
After a years-long stage of challenging experiments during which Prof. Avner Rothschild’s laboratory at Technion was unable to overcome the barrier in efficiency, he approached Drs. Yochelis and Visoly-Fisher to collaborate in the attempt to understand the missing piece of the puzzle.
“Beyond the scientific breakthrough, we have shown that the photo-electrochemical reaction mechanism belongs to a family of chemical reactions for which Prof. Gerhard Ertl was awarded with Nobel Prize in Chemistry, about a decade ago. Our discovery opens new strategies for photochemical processes, » says Dr. Yochelis of BGU’s Jacob Blaustein Institutes for Desert Research (BIDR) and the Alexandre Yersin Department of Solar Energy and Environmental Physics(YDSEEP).
The study, which was funded jointly by research grants from the Israel Ministry of National Infrastructures, Energy and Water, and the Israel Ministry of Science and Technology.
Published in Nature Communications[:]