For the last 20 years, researchers have focused on amyloid beta peptides and the “plaque” they sprout in diseased brains as the main target of Alzheimer’s research. But the pace of progress in treating — not to mention curing — the debilitating, neurodegenerative disease has been painfully slow.
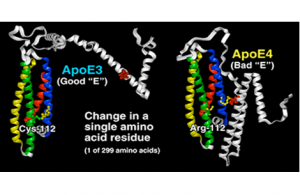
A Tel Aviv University study suggests a new target for Alzheimer’s research: the APOE gene. This gene, like Dr. Jekyll and Mr. Hyde, has two faces: a healthy form called APOE3 and a disease-related pathological form called APOE4. Researchers have developed a novel mechanism and approach with which to convert the “bad” APOE4 to the “good” APOE3.
The research was led by Prof. Daniel M. Michaelson, Director of the Eichenbaum Laboratory of Alzheimer’s Disease Research and incumbent of the Myriam Lebach Chair in Molecular Neurodegeneration at TAU’s Faculty of Life Sciences, together with Anat Boehm-Cagan, the Eleanore and Harold Foonberg Doctoral Fellow in Alzheimers Disease Research, and in collaboration with the commercial company Artery Ltd., based in California.

Pr Daniel Michaelson and Anat Boehm-Cagan
Focus on a new approach
“APOE4 is a very important and understudied target,” Prof. Michaelson said. “It is expressed in more than 60 percent of Alzheimer’s patients. Anti-APOE4 treatments are thus expected to have a major impact on the patient population.
“The normal APOE gene provides the interface that moves lipids — naturally occurring molecules that include fats, cholesterol, fat-soluble vitamins and other components essential to the health of cells — in and out of cells,” Prof. Michaelson continued. “Whereas the healthy APOE3 does so effectively, the bad form — APOE4 — is impaired.”
Prof. Michaelson and other groups found in past research that the bad APOE4 and the good APOE3 differed in their interactions with lipid cargo. The good APOE3, for example, is associated with substantially more lipids than APOE4.
The researchers devised an experimental approach to measure the “bad” features of APOE4, utilizing genetically manipulated mice expressing either good or bad forms of APOE. Mice with APOE4 exhibited impaired learning and memory, as well as damaged brain synapses and an accumulation of phosphorylated tau and a-beta molecules — two pathological hallmarks of Alzheimer’s.
Turning a bad gene to good
“Once this model was established and the pathological effects of APOE4 could be reproduced in mice, we could test therapeutic approaches and tackle APOE4 itself,” Prof. Michaelson said. “Because we know that APOE4 carries fewer lipids, we looked at the means of counteracting the lipidation deficiency.
“We focused on an enzymatic machinery called ABCA1 that loads lipid cargo onto APOE4. We found that the impaired lipidation of APOE4 could be successfully reversed by activating ABCA1. Most importantly, we discovered that this increased lipidation of APOE4 reversed the behavioral impairments and brain damage seen in non-treated APOE4 mice.”
The researchers found in the course of administering treatment that mice, which prior to the treatment exhibited disoriented behavior and seemed “lost,” were able following treatment to locate a submerged island in the middle of an artificial pond. Mice had forgotten familiar objects — like Coca Cola bottles — suddenly exhibited sharp object recognition.
“Is there really a magic bullet? One treatment that covers all aspects of Alzheimer’s? Not likely,” said Prof. Michaelson. “Therefore there is a need to define specific subpopulations and to develop treatments targeted at genetic risk factors of the disease, like APOE4, which affects more than half of the Alzheimer’s population.”
Publication in the Journal of Alzheimer’s Disease, september 2016