Ostéoporose, fractures : Bonus BioGroup (Israël) greffe des os autologues cultivés en laboratoire

[:fr]
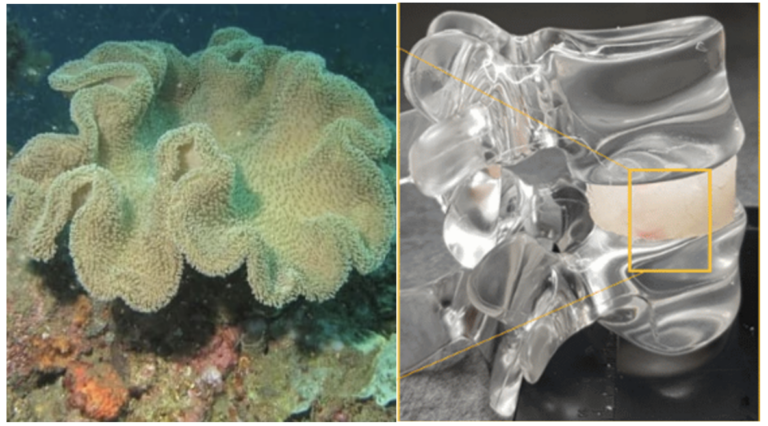
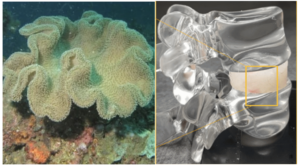
Non ce n’est pas de la science fiction. La société israélienne Bonus BioGroup a annoncé fin décembre 2017 avoir réussi pour la première fois une greffe dans la jambe d’un patient qui souffrait d’une perte osseuse sévère, greffon fabriqué en deux semaines par Bonus BioGroup et cultivé en laboratoire à partir des propres cellules dérivées de tissu adipeux du patient.
Bonus BioGroup est une société de biotechnologie, d’ingénierie tissulaire et de thérapie cellulaire personnalisée qui développe une technologie propriétaire de culture de greffons osseux humains destinés à des patients.
Auparavant, le patient, résident d’un Kibboutz, avait été opéré deux fois du tibia suite à un accident de voiture. Un système de fixation métallique des os avait été posé, mais une importante perte osseuse subsistait qui empêchait la cicatrisation complète de la fracture, limitant l’activité et provoquant un écrasement constant de l’os restant. L’opération a été réalisée par le Pr Nimrod Rozen, chef du service Orthopédie de l’hôpital Emek à Afula, entièrement affilié à la faculté de médecine du Technion.
Selon le Dr Nimrod Rozen, cette nouvelle chirurgie peut même aider les personnes qui souffrent d’ostéoporose, les patients atteints de cancer ou qui ont subi des amputations. Cela pourrait même être utilisé pour faire gagner quelques précieux centimètres à des personnes atteintes de nanisme.
Des examens pour participer aux essais cliniques
Les patients retenus pour l’essai clinique subissent une biopsie du tissu adipeux. La fabrication des cellules osseuses s’effectue dans le laboratoire de Bonus BioGroup en chambre stérile à Haïfa sur un échafaudage tridimensionnel biodégradable qui constitue un micro-environnement favorable à la croissance in vivo de l’os humain.
En laboratoire, les cellules graisseuses sont séparées des cellules capables de générer des tissus et des vaisseaux sanguins. Ces dernières sont cultivées dans un bioréacteur qui simule l’environnement à l’intérieur du corps humain et fournit des conditions optimales pour la génération osseuse.
Bonus BioGroup fabrique une greffe injectable composée de minuscules particules osseuses vivantes prêtes à être implantées dans la partie manquante de l’os du patient. Après la greffe, les particules d’os séparées se consolident en un tissu osseux autologue (donneur et receveur sont le même individu, par opposition à hétérologue) qui répare l’os blessé en six semaines.
En août 2017, Bonus BioGroup avait réussi pour la première fois une greffe d’os autologue dans l’avant-bras d’un patient souffrant d’une perte osseuse sévère, avec la même technologie. Lors d’un précédent essai clinique, Bonus BioGroup avait réalisé une percée révolutionnaire grâce à une injection unique de greffe osseuse humaine chez des participants souffrant de déficiences maxillo-faciales, tous sexes, âges ou contextes médical confondus, avec un taux de réussite de 95%.
Ces résultats n’avaient jamais été atteints à ce jour par aucun autre traitement médical. Un taux aussi élevé de réussite sur un petit échantillonnage, signifie que ce succès n’est pas une coïncidence, mais qu’il sera reproductible sur une plus grande population.
En parallèle, Bonus BioGroup a développé une seconde génération de greffe osseuse injectable qui permet de réduire le temps de fabrication, à moindre coût et sur une production de masse.
Des ambitions mondiales
Bonus BioGroup ambitionne d’être la première entreprise au monde à fabriquer et introduire sur le marché mondial de la rééducation osseuse (estimé à 7,5 milliards $ par an en 2017) une solution sûre et rapide pour combler les pertes osseuses grâce à une seule injection de greffe osseuse autologue après culture en laboratoire à partir de cellules prélevées chez le patient.
[:en]
Bonus BioGroup an Israeli biotechnology company engaged in personalized tissue engineering and cell therapy, and developing proprietary technology for growing live human bone grafts for implant in human patients, has announced, on December 19, 2017, that for the first time ever, a procedure was successfully performed for transplanting, in the leg of a patient who suffered from critical bone defect, a bone graft manufactured by the Company and grown outside of the patient’s body based on his own adipose (fat) tissue-derived cells.
Prior to implanting the bone graft manufactured by Bonus BioGroup in his body, the patient underwent surgery twice, in an attempt to mend a shinbone injured in an accident. Such surgery used a metal apparatus for connecting and fixating the fractured tibia. However, even after two operations a significant bone void remained in the shinbone, which prevented complete healing of the fracture, limited activity, and giving rise for constant concern of crushing remaining bone.
Under these circumstances, the alternate solutions that were considered for the patient, except for using the injectable bone graft manufactured by Bonus BioGroup included surgery for harvesting bone and its peripheral blood vessels from another part of his body, and implanting them in the area of the void, or alternatively, using an artificial bone substitute that shall not induce healing and not enable the patient to return to normal activity most likely due to failure of artificial bone substitute to integrate properly with the human body.
Every patient desiring to participate in the clinical trial for filling bone defect using a live implant of human bone manufactured by Bonus BioGroup is required to undergo examinations to verify suitability for participating in the clinical trial.
Patients verified as being suitable for participating in the clinical trial undergo biopsy of fat tissue for deriving various cells necessary for manufacturing a live human bone graft. The manufacturing procedure takes place at Bonus BioGroup’s manufacturing facility in Haifa, under controlled sterile conditions, on a three-dimensional biodegradable scaffold, in a unique environment simulating the conditions necessary for in vivo growth of human bone.
At Bonus BioGroup’s manufacturing laboratory, fat tissue is separated into comprising cells. While no use is made of fat cells, cells necessary for creating natural human bone are collected, from which the Company manufactures, within two weeks of sampling the patient’s fat tissue, an injectable bone graft made of tiny bone particles, ready to be implanted in the patient’s body. After transplantation the separated bone particles consolidate into solid, autologous bone tissue able to heal the injured bone.
On August 16, 2017, Bonus BioGroup announced that on the previous day, for the first time ever, a procedure was successfully performed for transplanting, in the forearm of a patient who suffered from critical bone defect, a bone graft manufactured by the Company and grown outside of the patient’s body based on his own adipose (fat) tissue-derived cells.
In a previous clinical trial, Bonus BioGroup achieved a revolutionary breakthrough in safe, rapid and efficient bone rehabilitation, through a single injection of live human bone graft, manufactured by the Company which was successfully demonstrated in all participants of the Company’s first clinical trial for repairing maxillofacial bone deficiencies in the upper or lower jaw, regardless of sex, age and medical background, recording a significance level of 95%.
Such extraordinary safety and efficacy results are not guaranteed by any other medical treatment. Demonstrating such high levels of statistical significance, even with a small sampling, means the success achieved is not coincidental, but rather is steadfast and repeatable, and therefore is expected to repeat itself also with a larger population.
Concurrently to conducting the first clinical trial, Bonus BioGroup has developed a second generation of injectable bone graft, which is more advanced, reduces manufacturing time, with less human involving, at a reduced cost and with suitability for mass production.
Bonus BioGroup strives to be the first company in the world to introduce to the global bone rehabilitation market, estimated at approximately US$7.5b per annum in 2017, a safe and rapid solution for filling bone defects, through a single injection of live autologous bone graft, manufactured by the Company after having been grown in the laboratory from cells sampled from the patient.
Currently, Bonus BioGroup is conducting two clinical trials for the second generation of its injectable bone graft, both defined Phase I/II, with the clinical objectives of evaluating safety and efficacy of using its injectable bone graft, as follows:
1. In September 2016, a second clinical trial began for filling bone void in the upper or lower jawbone, administered by Dr. Ephraim Tzur.
Up to 20 patients, male and female ages 18-70 are expected to participate in the second clinical trial for filling jawbone void.
2. In August 2017, a clinical trial began for filling critical bone defects in limbs – arms or legs; administered by Prof. Nimrod Rozen, head of orthopedics at Haemek Medical Center, Afula, according to an agreement between the Company and Clalit Health Services, Israel’s largest HMO.
The clinical trial for filling orthopedic bone defect shall be conducted for two indications: (1) extensive critical bone defect of long limb bone failing to mend, whether due to failure of the body’s natural rehabilitation process without medical intervention, or in the event of failure of orthopedic procedure to mend the limb; and (2) long limb bone extra-articular comminuted fracture.
The clinical trial for filling orthopedic bone defect is planned to include 30-40 participants, male and female, ages 18-75. In the first part of the trial, 10 participants shall be recruited for both clinical indications; in the second part, at least 10 more participants shall be recruited for each of the two clinical indications and up to 30 additional participants for both clinical indications together.
At the beginning of the first part of the clinical trial for filling orthopedic bone defects, the safety of using the second generation of injectable bone graft is examined in three participants suitable for the first indication. Such preliminary examination may be concluded within a few months.
Haemek Medical Center is one of the largest and most modern health centers in Israel, is fully associated with the Technion’s Faculty of Medicine, and operated by Clalit Health Services, Israel’s largest HMO providing medical services to more than 4.2 million individuals, which constitute a majority of the population of the State of Israel Medical observation of each clinical trial participant shall last six months from the date of implant.
Bonus BioGroup holds exclusive rights of use in six families of patents, which include two patents and ten patent applications. Bonus BioGroup is acting to register additional patents and patent applications, for realizing the revolutionary breakthrough in additional indications.
[:]