Technion : utiliser les séquences ADN d'URS comme antibiotiques

[:fr]

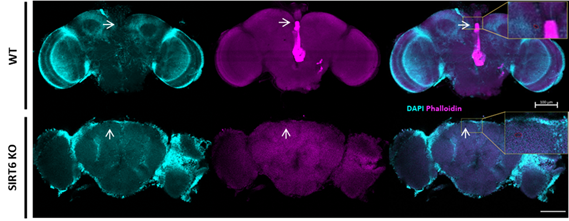
Le Pr Noam Adir du Technion a voulu mettre à profit l’accès aux séquences des génomes connus pour une recherche originale, celle des séquences absentes. Y a-t-il des séquences courtes sous-représentées par rapport à l’abondance à laquelle on s’attendrait à les trouver si leur distribution était aléatoire ? Le Pr Adir et son équipe, en particulier Dr. Sharon Penias-Navon et Tali Schwartzman, se sont alors mis à la recherche des séquences perdues qu’ils ont appelées URS pour under-representated sequences. Il s’agit de séquences d’ADN d’une douzaine de nucléotides qui sont très peu abondantes voire absentes d’un organisme donné. Le premier organisme étudié par les chercheurs a été la bactérie Escherichia coli. Ils ont observé que certaines séquences courtes d’ADN ne peuvent être lues par la machinerie cellulaire de cette bactérie pour être traduites en protéines. Quand un ribosome rencontre ces séquences, il s’arrête et la traduction est stoppée, ce qui affecte la croissance des bactéries.
Les URS sont spécifiques à une espèce donnée : celles qui interfèrent avec les ribosomes d’Escherichia coli laissent de marbre les nôtres. En fait, les nôtres ne semblent être arrêtés par rien, les chercheurs n’ayant pas trouvé d’URS humains. Ceci est un argument de plus en faveur d’une utilisation potentielle de ces URS comme antibiotiques, comme les chercheurs le suggèrent. Un brevet a déjà été déposé dans ce sens.
Publication scientifique dans PNAS, juin 2016
Source bvst, par Tirtsa Ackermann, doctorante, Université Hébraïque de Jérusalem
[:en]
A study from the lab of Prof. Noam Adir of the Schulich Faculty of Chemistry at Technion – Israel Institute of Technology: natural evolutionary processes prevent the presence of dangerous and potentially lethal molecular interactions by avoiding the presence of specific protein sequences in microorganisms. They found these sequences by a novel method – looking for what is missing in biological data sets. The group then experimentally showed that when these sequences are present in a protein, bacterial growth is indeed inhibited.
Evolution is an ongoing process, whereby those individuals of species that are the most fit for their environment have more offspring and thus out-compete less fit individuals. The individual’s fitness is a product of the quality of its cellular biochemistry, made possible by the thousands of enzymes that allow its physiology to perform all of the necessary chemical reactions that allow the cell to live. Deficiency in these molecular functions can lead to disease, loss of adaptability to environmental changes, or weakness against other organisms. The molecular machines that make life possible are large polymers made up of linear sequences of building blocks that contain different chemical functions: proteins, DNA, and RNA. Biological variety is a result of the evolutionary changes in these polymers, first and foremost the result of the astronomic number of possible permutations in the order of the 20 naturally occurring amino acid (AA) residues that are the building blocks of proteins. There are 8,000 possible sequences of three AAs, 160,000 sequences of four AAs, over 3 million sequences of five AAs and so on. Since proteins can contain between hundreds to thousands of AAs, the possibilities are endless.
The millions of different protein sequences found in all organisms determine the three-dimensional structures that give proteins the ability to function correctly. Proteins in cells can work alone or associate correctly with other cellular components, while avoiding incorrect and harmful associations with other components. Changes to the sequences naturally occur due to mutations (single site, or larger changes due to more dramatic sequence shuffling) of an organism’s DNA – the genetic material. Changes due to mutations can lead to new positive characteristics, or they may have negative consequences to the organism’s viability. A mutation that has a negative effect may prevent the organism from competing with other organisms in its environment, eventually leading to its demise. One could predict that over time, evolutionary pressure would work against the presence of organisms containing these internally lethal sequences and they would disappear.
Over the past few years, there has been a world-wide effort to obtain the entire DNA sequences (the entire genomes) of many organisms. These data have given us the ability to predict all of the possible protein sequences (the proteome) that might exist in organisms as simple as bacteria or as complicated as humans. Prof. Adir and his students, Dr. Sharon Penias-Navon and Ms. Tali Schwartzman, hypothesized that the huge amount of data made available by modern genomics would allow them to look for short sequences that occur less often than expected or are completely missing in the organism’s proteome. They developed a computer program that searched the many existing data sets to identify short sequences that are underrepresented (URSs). While they found that most of the sequences of three or four AAs indeed do exist at their expected frequency in the proteins of different organisms, URSs do exist. They used the program to search for URSs in the proteomes of many different organisms (especially pathogenic microorganisms) and found that different organisms have different URSs. Adir and Penias-Navon wanted to prove that these URSs are indeed harmful, and they hypothesized that protein synthesis (translation) by the ribosome is the function that URSs might harm.
They embedded bacterial URSs (identified in the proteome of the gut bacterium E. coli) comprised of three or four AAs in a normal protein sequence, and showed that no matter where they put the URS, protein translation was inhibited. They showed that these same E. coli URSs had no effect on protein translation in human cells, showing that the effect is species specific. They further showed that one four-AA URS was powerful enough to inhibit translation completely to the point where the growth of the bacterial cells was significantly reduced: these are indeed lethal sequences. Adir and Navon suggested that URSs could be used as highly specific anti-microbial agents, and a patent, together with the Technion, was submitted.
In order to obtain even more precise molecular details on the action of the URS, they initiated a collaboration with Prof. Joseph Puglisi and his student Dr. Guy Kornberg of Stanford University, who are experts in following protein translation in single ribosomes, thereby obtaining direct information on the translation reaction mechanism. Using these single molecule methods, the inhibitory effect of the existence of a URS on translation was confirmed. Their methods enabled a precise determination of the site of inhibition. They found that as soon as the URS AAs enter the entrance to the ribosomal nascent protein exit tunnel, translation is inhibited.
Publication in PNAS, June 2016
[:]