Potential for regenerative medicine and cancer research earns doctoral student Ido Sagi a Kaye Innovation Award
Stem cell research holds huge potential for medicine and human health. In particular, human embryonic stem cells (ESCs), with their ability to turn into any cell in the human body, are essential to the future prevention and treatment of disease.
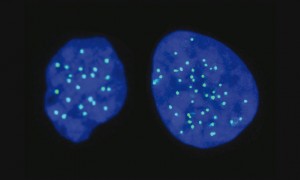
One set or two? Diploid versus haploid cells
Most of the cells in our body are diploid, which means they carry two sets of chromosomes — one from each parent. Until now, scientists have only succeeded in creating haploid embryonic stem cells — which contain a single set of chromosomes — in non-human mammals such as mice, rats and monkeys. However, scientists have long sought to isolate and replicate these haploid ESCs in humans, which would allow them to work with one set of human chromosomes as opposed to a mixture from both parents.
This milestone was finally reached when Ido Sagi, working as a PhD student at the Hebrew University of Jerusalem’s Azrieli Center for Stem Cells and Genetic Research, led research that yielded the first successful isolation and maintenance of haploid embryonic stem cells in humans. Unlike in mice, these haploid stem cells were able to differentiate into many other cell types, such as brain, heart and pancreas, while retaining a single set of chromosomes.
With Prof. Nissim Benvenisty, Director of the Azrieli Center, Sagi showed that this new human stem cell type will play an important role in human genetic and medical research. It will aid our understanding of human development – for example, why we reproduce sexually instead of from a single parent. It will make genetic screening easier and more precise, by allowing the examination of single sets of chromosomes. And it is already enabling the study of resistance to chemotherapy drugs, with implications for cancer therapy.

Diagnostic kits for personalized medicine
Based on this research, Yissum, the Technology Transfer arm of the Hebrew University, launched the company New Stem, which is developing a diagnostic kit for predicting resistance to chemotherapy treatments. By amassing a broad library of human pluripotent stem cells with different mutations and genetic makeups, NewStem plans to develop diagnostic kits for personalized medication and future therapeutic and reproductive products.
2017 Kaye innovation Award
In recognition of his work, Ido Sagi was awarded the Kaye Innovation Award for 2017.
The Kaye Innovation Awards at the Hebrew University of Jerusalem have been awarded annually since 1994. Isaac Kaye of England, a prominent industrialist in the pharmaceutical industry, established the awards to encourage faculty, staff and students of the Hebrew University to develop innovative methods and inventions with good commercial potential, which will benefit the university and society.
Ido Sagi received BSc summa cum laude in Life Sciences from the Hebrew University, and currently pursues a PhD at the laboratory of Prof. Nissim Benvenisty at the university’s Department of Genetics in the Alexander Silberman Institute of Life Sciences. He is a fellow of the Adams Fellowship of the Israel Academy of Sciences and Humanities, and has recently received the Rappaport Prize for Excellence in Biomedical Research. Sagi’s research focuses on studying genetic and epigenetic phenomena in human pluripotent stem cells, and his work has been published in leading scientific journals, including Nature, Nature Genetics and Cell Stem Cell.
NewStem, introduces a novel technology for predicting resistance to chemotherapy treatments. The Company is developing a diagnostic kit based on the pioneering research of Professor Nissim Benvenisty, the Azrieli Center for Stem Cells and Genetic Research, the Hebrew University of Jerusalem, in the field of human haploid pluripotent stem cells (HHPSCs)1. The novel technology will be presented at Yissum’s booth during the MIXiii Biomed 2017 Conference, to be held on May 23-25, in Tel Aviv, Israel.
Chemotherapy, still the mainstay in cancer treatment, often results in tumor primary and/or secondary resistance to the drugs, thereby not only rendering the therapy ineffective, but also subjecting patients to harsh and potentially harmful treatments. Pluripotent stem cells, cells that are able to differentiate to any given cell type, are used for a variety of applications, from basic research to therapeutics. Due to their differentiation capacity, one potential use of stem cells is to assess the influence of mutated genes on a variety of cells, physiological processes and drug responses. However, since these cells, as most human cells, contain two copies of each gene (diploid cells) it is often difficult to generate cells in which both copies of the relevant genes are mutated.
The technology being developed by NewStem enables the generation of fully functional stem cells containing only one copy of each gene (haploid cells). The Company, recently founded as a spinoff of Yissum Research Development Company of the Hebrew University of Jerusalem, the technology-transfer company of the Hebrew University, possesses proprietary intellectual property, reagents and experience related to the isolation and differentiation of haploid pluripotent stem cells (HPSCs), their genetic manipulation, immunogenicity, tumorigenicity and their unique capacity in disease modeling. A unique and broad library of HPSCs with different mutations and genetic makeups enables NewStem not only do develop diagnostic kits for personalized medication, but also, in the future, to develop therapeutic and reproductive products.
“The haploid stem cells we’re developing have the potential to change the face of medical research as they hold a pivotal role in regenerative medicine, disease therapy and cancer research. NewStem is the only company worldwide to develop novel products based on this paradigm-changing proprietary technology, which we acquired under an exclusive license from Yissum,” commented Ayelet Dilion-Mashiah, CEO of NewStem. “We are currently in the process of raising funds for our research and development and regulatory activities.”